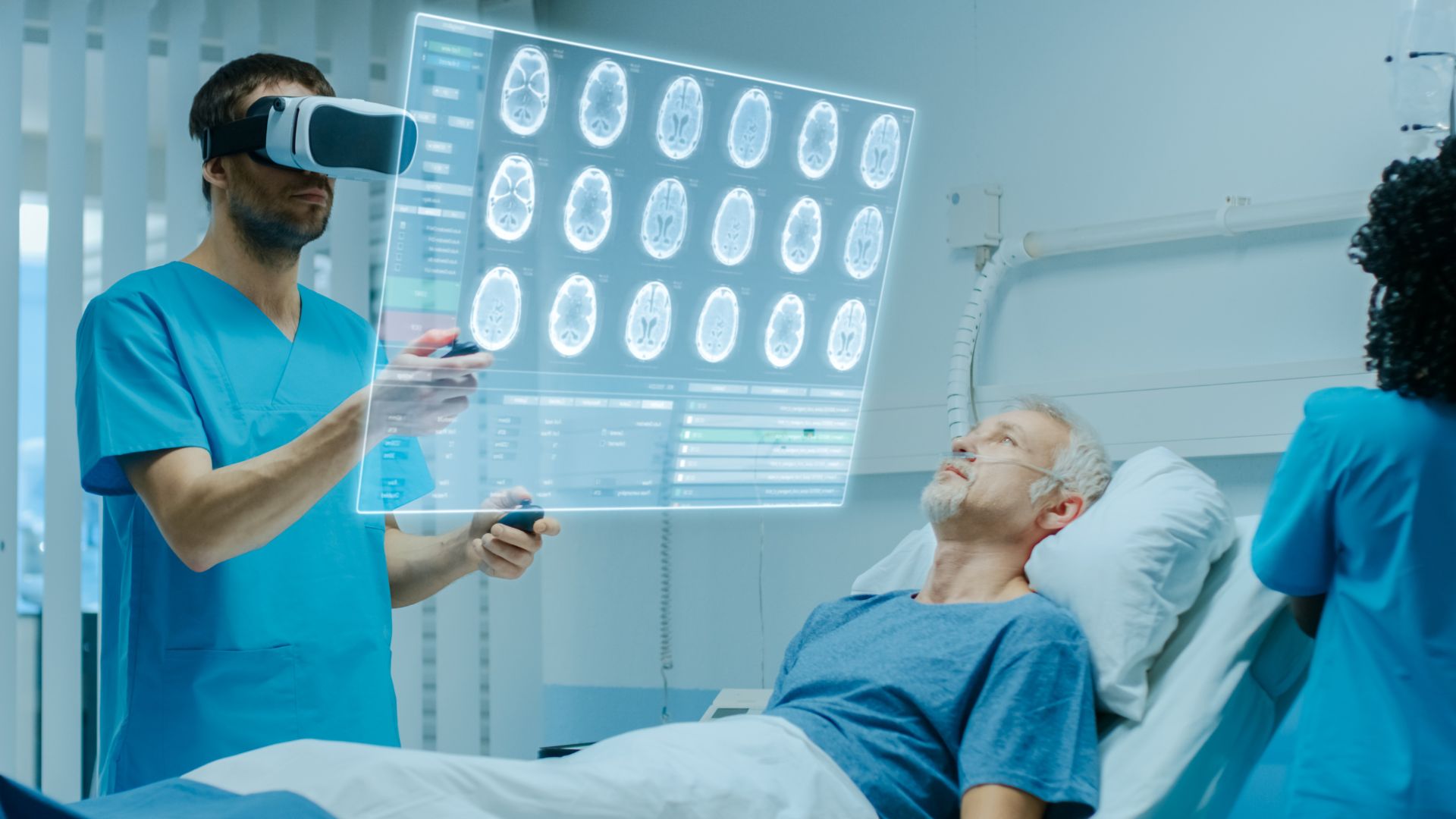
You could start selling or exporting a product but wait! Within the regulator’s letter, there may be clauses that require critical assessment and managing the post-marketing compliance necessities. Certain terms would apparently leave the decisions to you. You might need assistance in deciphering the implications of:“although, be aware, if, may, however, etc.”.
It is interesting to see an example:
“If your device is classified into either class X or class XX, it may be subject to additional controls”. Exactly what additional controls?
“Although this letter refers to your product as a device, please be aware that some cleared products may instead be combination products”. When should you do the assessment?
“The general controls provisions of the Act/Rules include requirements for GMP/ISO compliance, labeling compliance, and prohibitions against misbranding and adulteration”. Simply decoded – lots of internal controls!
“We remind you, however, that device labeling must be truthful and not misleading”. Requires continued risk assessment and compliance!
The Regulator may publish further announcements concerning your device through new Acts/Rules. You need to monitor regulatory changes and compliance with changes!