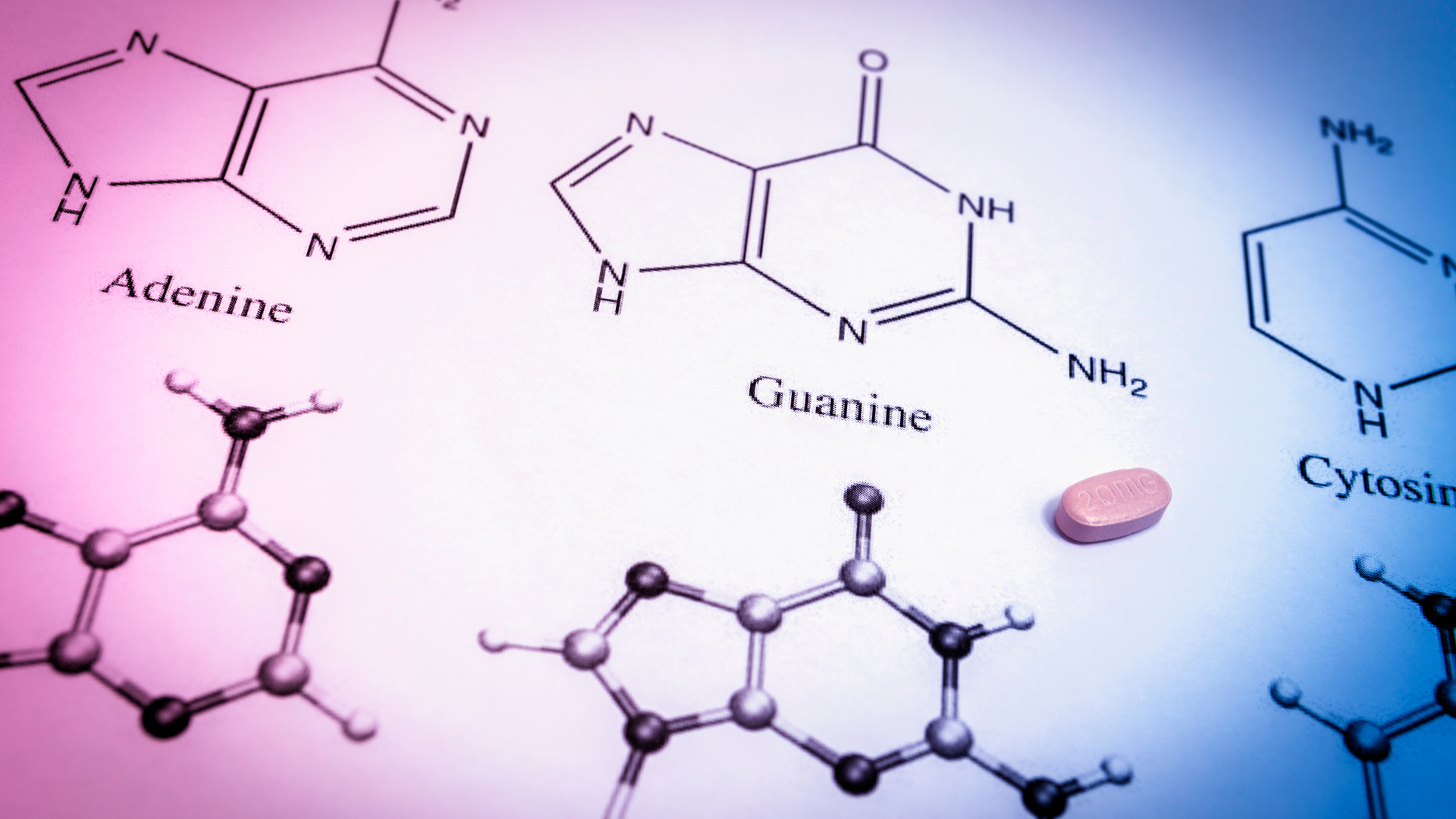
Our pharmacology document assistance involves an in-depth review of material substances that are critical to the action of the product and includes drug substance Information, drug formulation information, an analysis of excipients (pharmacopeial or novel), clinical impact of impurities/degradation products, and other essential parameters that might impact efficacy or safety of the product. Our review may involve an in-depth analysis of data generated by the company along with information available from published literature, well well-accepted reference textbooks.
For evidence on primary and secondary pharmacology, pharmacokinetics/ADME/toxicokinetics we study the data generated by the company. The company may have already conducted some in vitro or in vivo studies as part of early developmental activities to establish the safety, mechanism of action, or efficacy of the product and to support its intended use. If the product development is in the concept stage, we provide guidance on appropriate and acceptable early scientific studies that might be required to support the filing of IND/NDA/BLA at a later stage.